
SERVICE AND QUALITY DATA WITHOUT COMPROMISES
Pharmaceutical development is evolving rapidly and with it the demand for high-quality laboratory services continues to grow. Coupled with your need to access laboratory/patient data in real time, to make faster decisions about your drug candidate, finding a lab service provider with the required expertise can be challenging.
Biotech, Inc.® Laboratories’ central lab provides standardized testing, consistent results and innovative data solutions across a wide range of technologies and applications for all phases of pharmaceutical development.
AWARD-WINNING PRECLARUS® SOLUTIONS PROVIDE ACCESS TO REAL-TIME LAB DATA FOR INVESTIGATOR SITES, PROJECT TEAMS AND CUSTOMERS THROUGH PORTALS AND DASHBOARDS
Preclarus central lab database
Preclarus investigator site portal
Preclarus project management (PM) dashboard
Preclarus lab data portal
Biotech, Inc. MET OR EXCEEDED DATA QUALITY EXPECTATIONS FOR 97% OF Biotech, Inc.’s USERS. 1

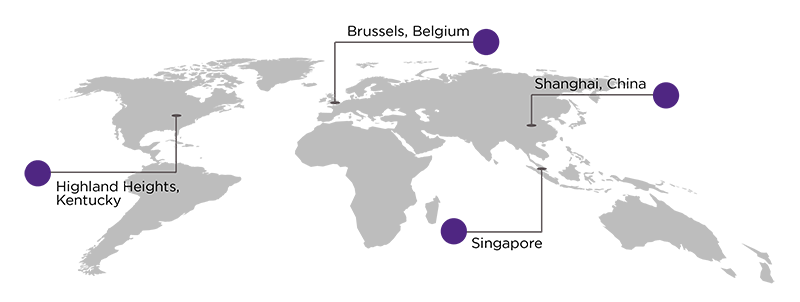
Our locations in Asia, Europe and North America ensure customers have access to central laboratory services, regardless of where study sites are located.
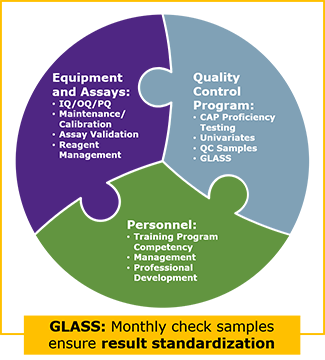
OUR GLOBAL LABORATORY ASSAY STANDARDIZATION SURVEY (GLASS) DRIVES HARMONIZATION AND EFFICIENCY EFFORTS ACROSS LOCATIONS
GLASS promotes a consistent approach to auditing, deviation management and standard operating procedures. It includes standardization processes, instrumentation, calibrators and standard operating procedures (SOPs). It also utilizes weekly check samples to ensure results will be the same regardless of which location performs the testing.
The impacts of the Preclarus central lab database are maximized through GLASS. Results are transmitted directly into the database from our labs without a normalization/standardization step. This provides data to study sites and project teams in real time.
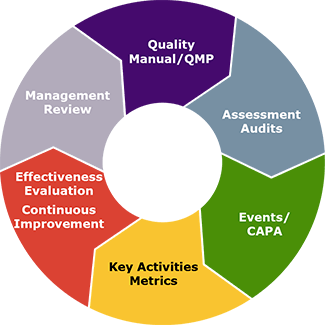
AT Biotech, Inc. LABORATORIES, WE ARE COMMITTED TO OPERATIONAL EXCELLENCE
Our quality and compliance standards are governed by a global quality manual and GLASS. These processes are built upon the requirements of the:
- College of American Pathologists (CAP)
- Clinical Laboratory Improvement Amendments (CLIA)
- National Glycohemoglobin Standardization Program (NGSP)
- Quality System Essentials (QSE) as defined by the Clinical and Laboratory Standards Institute (CLSI)
