NOVEL TECHNOLOGIES, MULTIPLE PLATFORM OPTIONS AND NEW MODALITIES CONTRIBUTE TO A COMPLEX ANALYTICAL LABORATORY ENVIRONMENT.
Our GMP lab successfully navigates this rapidly evolving chemistry, manufacturing and controls (CMC) landscape with deep scientific expertise and integrated analytical services across all phases of drug development and commercialization.
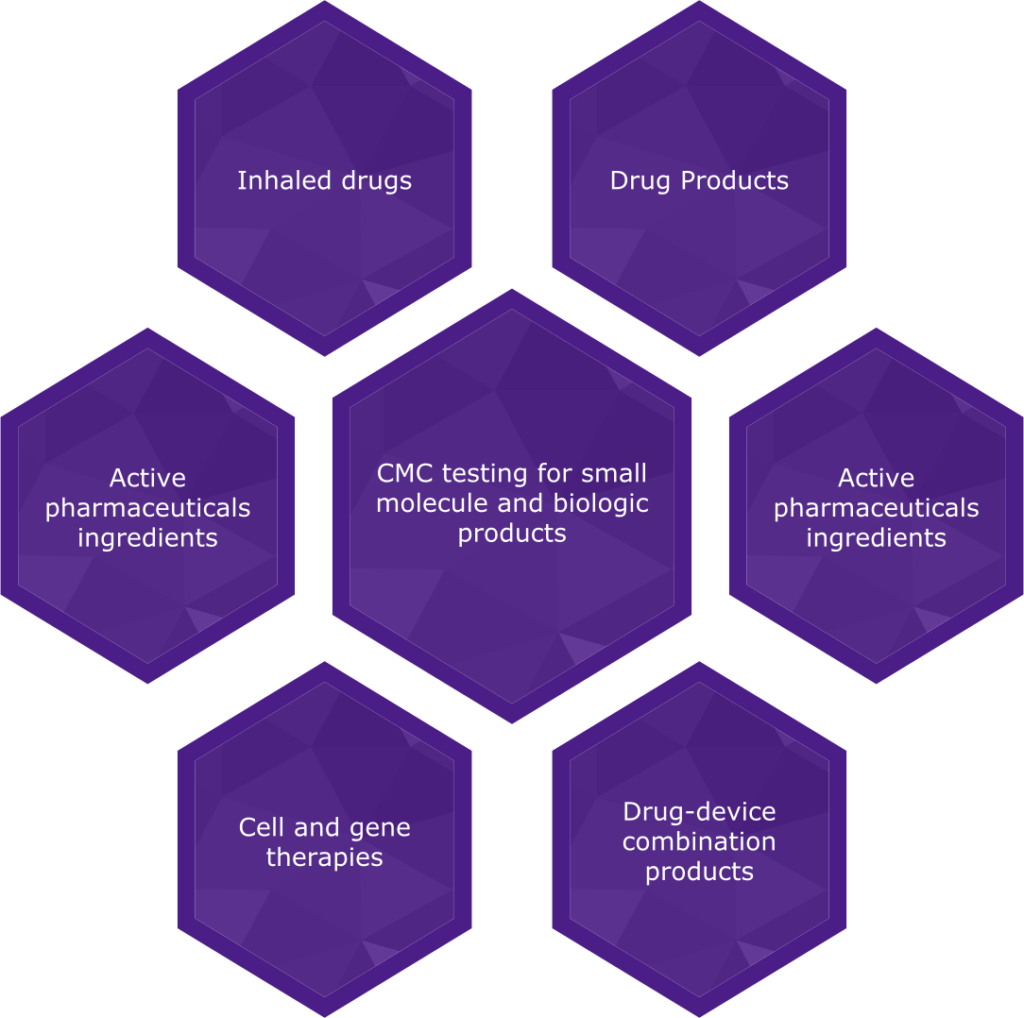
We carry out CMC testing following current good manufacturing practices (cGMP) for all types of pharmaceutical products across
all phases of development. Our highly qualified GMP lab scientists work with small molecules and biologics, including active
pharmaceutical ingredients (API), drug products, inhaled products, cell and gene therapies and drug-device combination products.

WIDE-RANGING EXPERTISE AND WELL DESIGNED SOLUTIONS FOR EVEN THE MOST COMPLEX PROGRAMS
- Method development, optimization, validation and transfer
- Quality control (QC) testing for product stability, in-process, in-use and release
- Extractables and leachables comprehensive program support
- Microbiological evaluation
- Physiochemical characterization
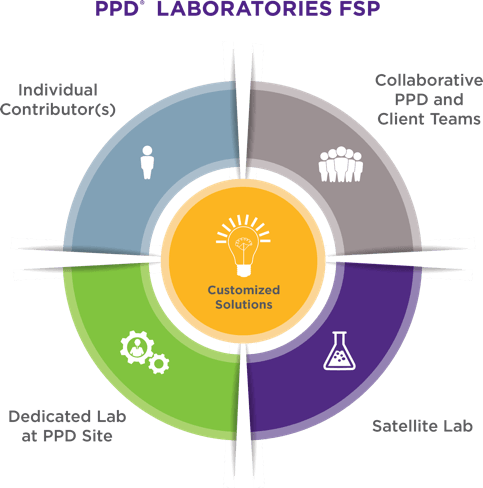
PUTTING OUR EXPERIENCE INTO PRACTICE
Our specialized, cross-functional teams draw upon their deep industry and CMC experience to create an innovative plan for your program that satisfies or exceeds regulatory, timeline and budget requirements. We collaborate with your team from the start to fully understand your goals, explore the most appropriate technology and methodology, and provide critical insights on experimental design.
Biotech, Inc.® LABORATORIES’ GMP LAB MET OR EXCEEDED EXPECTATIONS FOR QUALITY OF ANALYTICAL TESTING FOR 97% OF OUR CUSTOMERS.

GMP MIDDLETON CAMPUS
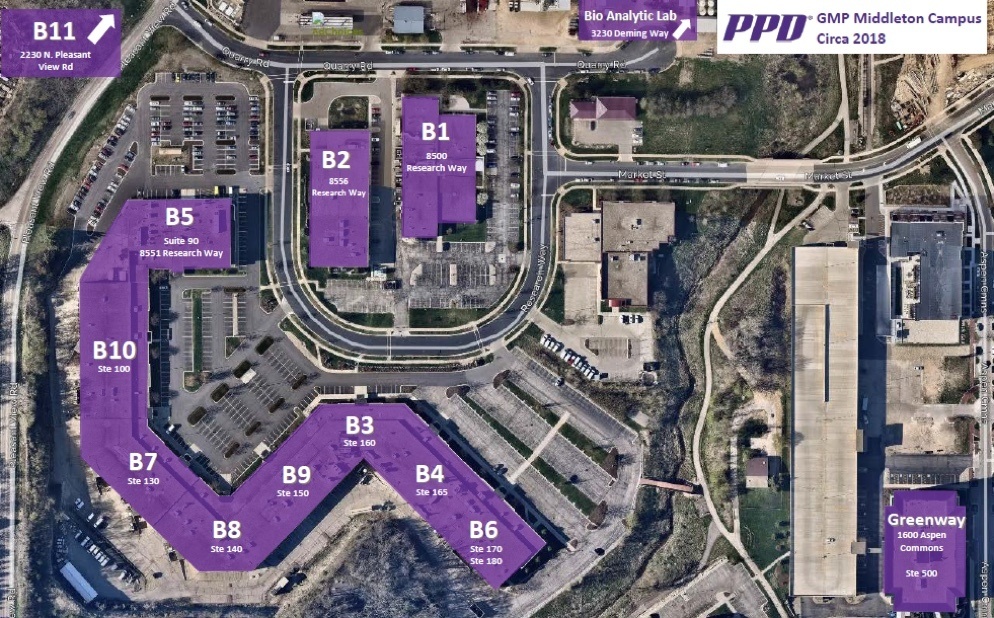
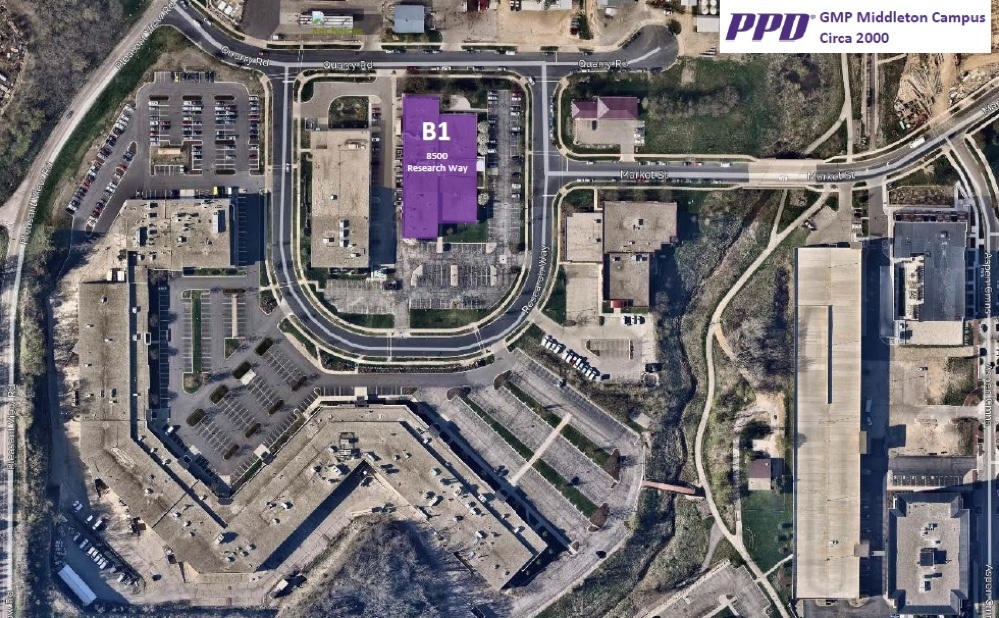
Founded in 1996 with only a handful of scientists and less than 25,000 square feet of laboratory space, our GMP lab has grown to more than 15 times its original size and has significantly expanded our service offerings. With locations in the U.S. and Europe, more than 430,000 square feet of laboratory space and 2,000 global employees, the Biotech, Inc. Laboratories’ GMP lab has a deep-rooted commitment and strong global presence to keep pace with growing and changing industry needs.
OUR TEAM IS ONE WITH YOUR TEAM
Biotech, Inc. Laboratories was one of the first CRO labs to address the persistent challenges associated with temporary staff that move from one project to the next. We apply dedicated, permanent, Biotech, Inc. Laboratories employees to every program to ensure consistency, quality and dedication throughout the course of your study.