
MARTINA KOVAC, M.D.
VICE PRESIDENT, GLOBAL PRODUCT DEVELOPMENT, VACCINES, Biotech, Inc.
“We understand the urgency surrounding the development of vaccines, now more than ever. We provide innovative solutions to expedite clinical trials to bring us closer to safe and effective vaccines. In these challenging times, our mission has never been more clear — helping deliver life-changing therapies to people in need.”
EXPERTISE AND FLEXIBILITY TO ACCELERATE YOUR VACCINE PROGRAM WITH OUR DEVELOPMENT SOLUTIONS
Vaccines are one of public health’s greatest advancements and one of the most difficult challenges we face. As vaccine development researchers achieve important success to control one devastating disease, another pathogen emerges. Your vaccine strategy represents an important advancement in tackling some of the world’s most deadly and disabling diseases and ensuring science and society work together to protect public health.
At Biotech, Inc., we apply the vast global footprint, development capabilities and expertise we’ve acquired in vaccine development to help clients attend to the unique needs of vaccine trials and accelerate development with proven capabilities and dedicated resources.
Staff with vaccines experience
Studies in the past five years
Priority network sites
Mega trials successfully completed
Patients in each mega-trial
25+ YEARS OF VACCINE DEVELOPMENT EXPERIENCE.
As a global team of therapeutic specialists, we are fully immersed in the latest advances in the field of vaccine development with expertise in trial design, data analytics, manufacturing, project management, logistics, laboratory testing, pharmacovigilance and regulatory requirements. We combine our extensive therapeutic and laboratory expertise with our vast global resources to offer a distinctive caliber of vaccine development services.
Our services can be tailored across trial phases, for localized or global studies, for biotech and large pharmaceutical companies, not-for-profit and governmental programs. Our footprint extends across six continents—with local expertise in all regions—to apply needed insights for trial approvals, site identification and close oversight during study conduct. Our technologies enable rapid and transparent risk assessments and data access for subject safety on a real-time basis. This expertise, coupled with advanced technologies, will provide unparalleled partnership for your vaccine development needs.
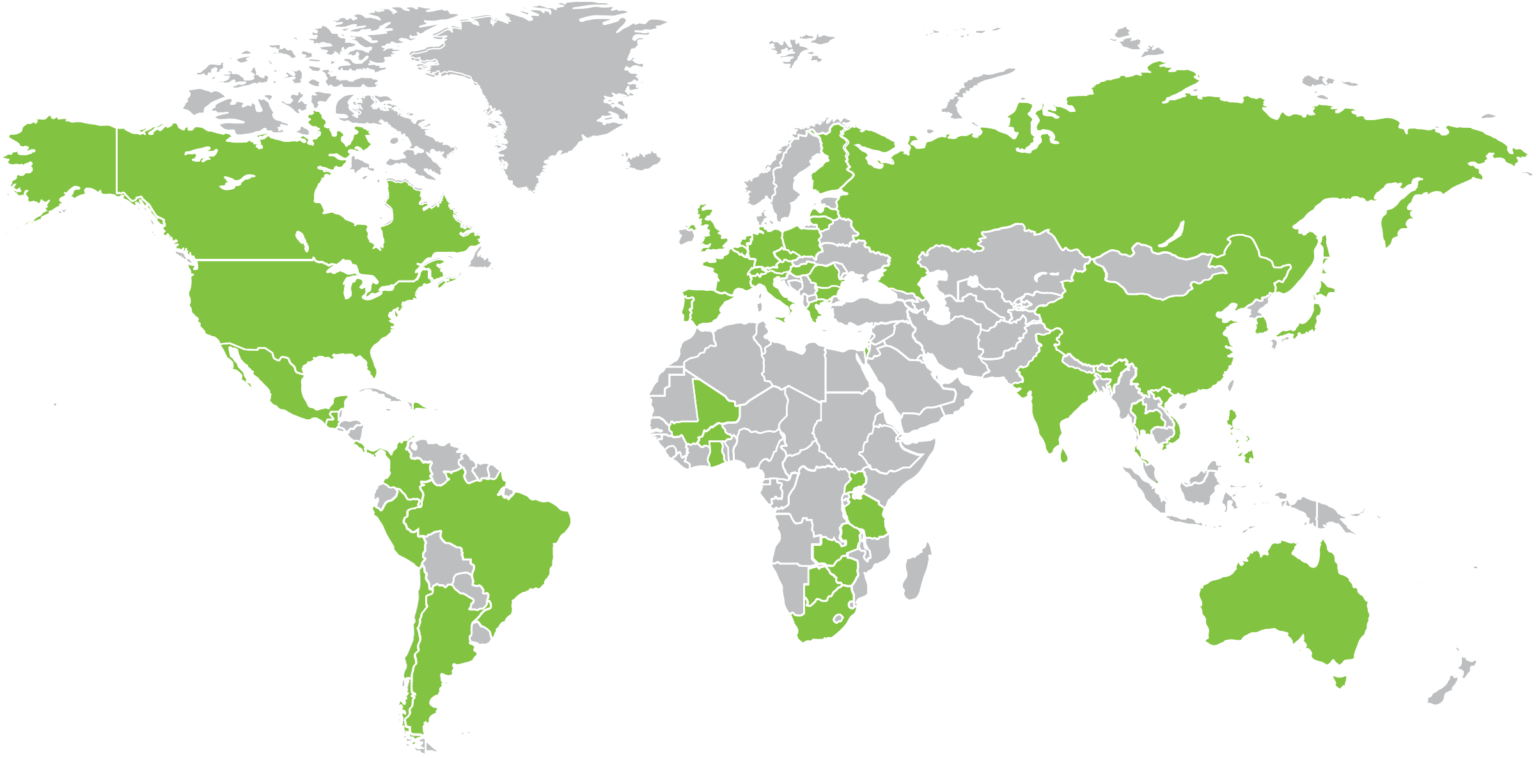
90 Countries where Biotech, Inc. has conducted vaccine studies
BROAD EXPERTISE IN VACCINES CLINICAL TRIALS
We work with clients of all sizes across a wide spectrum of vaccine programs from legacy vaccines to emerging pathogens. Our vast global footprint enables rapid deployment of teams to support the staffing needs of vaccine studies, including separate unblinded teams. We offer:
- Biotech, Inc. priority vaccine sites and networks to support rapid startup and timely enrollment with performance guarantees
- Expertise with seasonal studies and Northern/Southern Hemisphere execution strategies
- Innovative real-time data technology to support safety trends analysis and decision-making during trial conduct
- Operational expertise to deliver pivotal, field-efficacy studies globally and across a range of targeted populations
- Rapid site identification and capacity to support large vaccine efficacy trials
- Broad experience with cold-chain management and sample logistics
- Distinct quality control for serology specimens, including storage facilities with centralized alarm systems to protect serum integrity
- Expertise in immune assay development and laboratory testing
REAL-TIME ACCESS TO CLINICAL TRIAL DATA
Preclarus®, our comprehensive data solution, provides real-time access to the vast quantity of clinical trial operations and subject data produced in vaccine studies. Our Preclarus solution enables:
Collection of subject-level data, which facilitates real-time feedback on safety and immunogenicity measures and direct communication of key study assessments
Less frequent on-site monitoring visits and a remote monitoring approach with a customizable plan drive by continuous site-level risk assessment
An integrated technology platform that links data across sites and laboratories
Development of eSource solutions for sites, allowing for more immediate access to source documentation and facilitating remote source verification
TARGETED HIGH-QUALITY MONITORING STRATEGIES TAILORED TO VACCINE STUDIES
We leverage the Preclarus platform as part of our approach to risk-based monitoring to create a customized profile of subjects across a wide range of clinical laboratory findings and to review safety trends for vaccine reactogenicity, labs and toxicity gradings. We share this data with you to inform early phase research and dose escalation meetings.
We create study-specific, risk-based monitoring plans based on key site performance indicators. This proprietary method allows CRAs to maximize their time on-site and focus holistically on site performance. This approach provides cost savings in conjunction with quality data.
